Vivatmo: FeNO for Clinical Studies
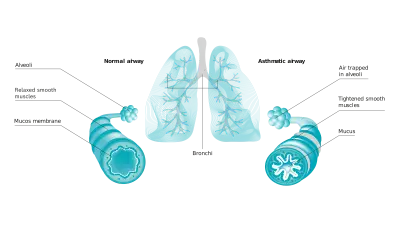
FeNO is a well-established biomarker for asthma. Not only doctors and patients, but also clinical researchers and Contract Research Organizations (CROs) can greatly benefit from FeNO home measurement with the Vivatmo system from Bosch.

FeNO Home Monitoring – Benefits for Clinical Studies
The Vivatmo system from Bosch provides simple and precise FeNO measurement in medical practices and at home. Clinical researchers and CROs conducting studies with FeNO benefit from Vivatmo home monitoring:
Cost reduction
Lower the cost of data collection, such as reducing on-site visits and device costs.
More data over time
Capture more FeNO values during the given observation period while maintaining high data quality.
Boost clinical insights
Enrich data with app-recorded context information and raise clinical insights to the next level.
Joy of Use: Higher Adherence, Fewer Drop-Outs
Vivatmo me is the world’s first device approved for FeNO home monitoring. Together with the Vivatmo app, it makes home testing quick, easy, and highly reliable. The system guides users step by step, making incorrect measurements virtually impossible. Readings are automatically transmitted via Bluetooth to the Vivatmo app or to third-party systems such as a CRO’s study app. Patients can also log relevant data such as symptoms, medication, or well-being. The workflow is designed to make FeNO home monitoring simple and joyful, promoting high adherence and providing study teams with complete, reliable datasets.

The Vivatmo System for Clinical Studies
The Vivatmo system is ideally suited for conducting clinical studies. It consists of two devices: Vivatmo pro, the FeNO measurement device for use in study centers. And Vivatmo me, the world’s only FeNO home measurement device for patients. The FeNO values measured at home are transferred via Bluetooth either to the Vivatmo app or to any third-party app using the Software Development Kit (SDK). The Vivatmo app offers the export of reports (CSV or PDF) to obtain an overview of all relevant data.
Easy Integration of FeNO and Context Data
Vivatmo streamlines data integration for clinical researchers and CROs. It enables easy consolidation of data from study centers and home monitoring into a central study database. The Vivatmo app and SDK facilitate transmission of FeNO values and context data from home monitoring to the researcher's database. For easy integration with healthcare IT systems, Vivatmo pro offers an HL7 and a GDT standard-based interface. This eliminates the need for manual data entry and reduces potential errors.

References
Beeh et al. 2025: Device adherence and user experience with daily home-based FeNO monitoring (Vivatmo me) in asthmatics: the FeNO@home observational study. BMC Digital Health 2025: Usability assessed via User Experience Questionnaire Plus: Patients rated usability in several categories on a scale from –3 (very poor) to +3 (excellent). The median UEQ+ score was 2.05. Adherence measured as the proportion of study days with at least one FeNO measurement. The median Proportion of Days Covered (PDC) across all patients was 93%. Physicians rated expectation fulfilment on a scale from 0 (not at all) to 6 (absolutely); 87% of responses were 5 or 6. DOI: 10.1186/s44247-025-00212-1
This website contains general product information about the Vivatmo system from Bosch. Not all products and their functions mentioned here are approved in every regional market. For a detailed description of the products and features as well as information on intended and safe use, please refer to your locally authorized Bosch distribution partner and the instructions for use that are valid for your country.